

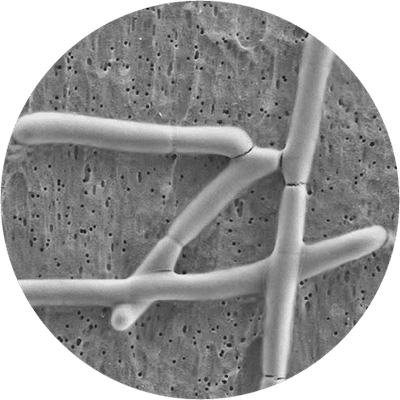
This is a gram positive, non-sporulating, anaerobic, rod shaped bacteria, which is a natural inhabitant of the human gut and a member of the Lactobacillus acidophilus species. Lactobacillus acidophilus LA-05® is known to produce lactic acid, acetic acid and hydrogen peroxide; these metabolites make the intestinal environment less favourable for the growth of potentially pathogenic microorganisms.
It is important to note that this strain has been predominantly trialled alongside Bifidobacterium lactis BB-12®, where it has been shown to help support a variety of different gastro-intestinal symptoms, including those caused by inflammatory bowel disorders. There have also been encouraging results from studies looking at antibiotic-associated side effects; immune function, glucose tolerance and lactose intolerance.
The strain Lactobacillus acidophilus LA-05® is a food supplement, often studied alongside the strain Bifidobacterium lactis BB-12®. In one such study safety was assessed as a key endpoint. It found administration of L. acidophilus LA-05® and B. lactis BB-12® to have satisfactory safety, as measured by recording adverse events, and good tolerability, as measured using a global wellbeing scale (Chatterjee, S. et al., 2013).
L. acidophilus LA-05® has been recovered in stool samples after oral administration, demonstrating its ability to survive to reach the gut alive. In one study it was administered in the form of food (a yoghurt) to healthy adults for 4 weeks, after which L. acidophilus LA-05® was found to be present in stool analysis, indicating it had survived to reach the gut alive (Savard, P. et al., 2011). As in the study above, the survival of B. lactis BB-12® was also assessed in this trial.

Diarrhoea is a common health problem which can be caused either by food or water infected with pathogens such as Escherichia coli, Salmonella, Shigella or Campylobacter. Several studies have suggested that it may be best to recommend certain probiotic strains over others in order to help alleviate this symptom (McFarland L.V., 2005), and the most extensively researched strains are featured in this database.
In travellers going to Egypt, a probiotic supplement including Lactobacillus acidophilus LA-05® and Bifidobacterium lactis BB-12® significantly decreased the incidence of diarrhoea in the test group compared to the placebo group (Black et al, 1989).

Diarrhoea may also be caused by antibiotics, which can have an indiscriminate action on the gut flora, wiping out both beneficial and pathogenic bacteria. This disruption may allow pathogenic bacteria to flourish and overgrow, causing unpleasant symptoms such as diarrhoea. The ‘triple-therapy’ antibiotic treatment for H. pylori is particularly harsh on the digestive system, so this was the focus of a study by Sheu et al (2002), where 160 patients infected with H. pylori were randomised into two groups. One group was treated with a triple therapy as well as a probiotic yoghurt containing Lactobacillus acidophilus LA-05® and Bifidobacterium lactis BB-12® (AB-yoghurt), and the other only received the triple therapy. The probiotic group continued to take the yoghurt for four weeks after the triple therapy treatment ceased. Eight weeks later, patients were assessed for the success of H. pylori eradication using stool samples before and after the treatment. The results indicated that the treatment plus yoghurt group had a higher eradication rate for H. pylori than the antibiotic-only group, and showed a restored level of Bifidobacteria in the gut at week five to pre-treatment level (Sheu et al, 2002).
Further Relevant Studies: Black F.T. (1996), Sheu et al (2006), Shioya M, et al (2000a) Shioya M, et al (2000b), Wang et al (2004).
The Lactobacillus acidophilus LA-05® and Bifidobacterium lactis BB-12® combination has been shown to help alleviate chronic constipation in elderly patients: in this study, the participants either received unfermented milk or fermented milk containing the probiotics. The group who received the probiotic milk experienced a significant improvement in frequency of bowel movements compared to baseline (Alm L, 1993).
A supplement containing Lactobacillus acidophilus LA-05® and Bifidobacterium lactis BB-12® was given daily for 4 weeks to 51 Ulcerative Colitis (UC) patients and 10 patients with Familial Adenomatous Polypeptosis (FAP), all of whom had received ileal-pouch-anal-anastomosis surgery. Symptoms of UC activity were significantly decreased during intervention in the UC group as well as the FAP group. The researchers concluded that this probiotic combination had a positive effect on symptoms and inflammation in UC patients operated on with IPAA (Laake K.O, et al, 2005).
Other studies have shown that supplementing with L. acidophilus LA-05® and B. lactis BB-12® can lead to a faster normalisation of the gut flora after taking antibiotics. A double-blind, placebo-controlled study looked at 20 participants who were receiving antibiotics, plus either a placebo or probiotic capsules (4 billion CFU) for 7 days, and then 14 days after antibiotics treatment had ceased. It was found that the intestinal tract recolonised more quickly in the probiotic group than the placebo group. Also, adverse effects of the antibiotics were only seen in 1 person in the probiotic group as opposed to 3 people in the placebo group (Black et al, 1991).
Further relevant studies: Fox et al (2015), Nord CE et al (1997).
The efficacy of the probiotic combination was tested in 50 lactose intolerant adults, who had been on a lactose free diet. The subjects were given probiotic capsules containing the strains Lactobacillus acidophilus LA-05® and Bifidobacterium lactis BB-12®, whilst gradually increasing the amount of lactose in their diet. The results indicated that 80% of the participants considered their well-being either unchanged or improved, rather than feeling the effects of lactose intolerance (Virta et al 1993).
Further relevant studies: Jiang et al (1996), Lin MY (1991).
A total of 39 patients undergoing major elective colonic resection were randomised to receive pre-, peri- and post-operative optimisation or conventional peri-operative care. Optimisation with a probiotic supplement including the strain L. acidophilus LA-05® was associated with a significant shorter hospital stay, quicker recovery of gut function, and shorter requirement for a catheter and intravenous infusion compared to conventional care without a probiotic supplement (Gatt et al, 2005).
Further relevant studies: Anderson (2004), Sagen (1989), Wildt S, et al. (2006).
Type 2 diabetes is often associated with gut dysbiosis. In this trial 50 volunteers with type 2 diabetes were divided into two groups, one group were given a fermented milk containing Lactobacillus acidophilus LA-05® and Bifidobacterium lactis BB-12®, while the control group were all given a placebo. Anthropometric measurements, body composition, fasting blood and faecal samples were taken at baseline and after 6 weeks. The conclusions were that the probiotic milk benefited those with type 2 diabetes (diabetes mellitus) by improving their glycaemic control, however, the intake of fermented milk also seems to be involved with others metabolic changes, such as decrease in inflammatory cytokines (TNF-α and resistin) and increase in acetic acid (Tonucci et al, 2017).
In-vitro and in-vivo studies have shown that Lactobacillus acidophilus LA-05® can be associated with nonspecific stimulating effects on the production of cytokines and phagocytic activity as well as antibody production (Hatcher, 1993); (Miettinen et al, 1996); (Tejada-Simonet et al, 1999).
Authors: Information on this strain was gathered by Joanna Scott-Lutyens BA (hons), DipION, Nutritional Therapist; and Kerry Beeson, BSc (Nut.Med) Nutritional Therapist.
Last updated - 21st May 2020
As some properties & benefits of probiotics may be strain-specific, this database provides even more detailed information at strain level. Read more about the strains that we have included from this genus below.
Lactobacillus acidophilus strains: Lactobacillus acidophilus NCFM®, Lactobacillus acidophilus Rosell-52.
Lactobacillus casei strains: Lactobacillus casei Shirota, Lactobacillus casei DN-114001.
Lactobacillus plantarum strains: Lactobacillus plantarum LP299v.
Lactobacillus reuteri strains: Lactobacillus reuteri Protectis and Lactobacillus reuteri RC-14®.
Lactobacillus rhamnosus strains: Lactobacillus rhamnosus LGG®, Lactobacillus rhamnosus HN001, Lactobacillus rhamnosus GR-1® and Lactobacillus rhamnosus Rosell-11.
Lactobacillus paracasei strains: Lactobacillus paracasei CASEI 431®.
For products containing these strains visit the Optibac Probiotics shop.
For more information and the latest research on probiotics, please visit the Probiotic Professionals pages.
Alm L, et al., (1993) ‘Effect of a new fermented milk product "CULTURA" on constipation in geriatric patients. 1st Lactic Acid Bacteria Computer Conference Proceedings’. Horizon Scientific Press, Norfolk, England 1993.
Anderson, A et al., (2004) ‘Randomised clinical trial of synbiotic therapy in elective surgical patients’. Gut, 53, 241-245.
Black, F. et al., (1989) ‘Prophylactic efficacy of Lactobacilli on traveller’s diarrhoea’. Travel Med. 1989, 333-335.
Black, F. et al., (1991) ‘Effect of lactic acid producing bacteria on the human intestinal microflora during ampicillin treatment’. Scandinavian Journal of Infectious Disease 23(2):247-54.
Black, F. (1996) ‘Placebo-contolled double-blind trial of 4 Lactobacilli strains (HIP) used as prophylactic agent against traveller's diarrhea (2 trials)’. Report by G. Nirnberger, Bioconsult, GmbH, Austria.
Chatterjee, S. et al., (2013) ‘Randomised placebo-controlled double blind multicentric trial on efficacy and safety of Lactobacillus acidophilus LA-5 and Bifidobacterium BB-12® for prevention of antibiotic-associated diarrhoea’. J Assoc Physicians India, 61 (10): 708-12.
Fox, MJ et al., (2015) ‘Can probiotic yogurt prevent diarrhoea in children on antibiotics? A double-blind, randomised, placebo-controlled study’ BMJ Open, 2015; 5. 2015; 5:e006474.
Gatt M,et al., (2005). ‘Randomized clinical trial of multimodal optimization of surgical care in patients undergoing major colonic resection’. British Journal of Surgery, 92:1354-1362.
Jiang T, et al., (1996) ‘Improvement of lactose digestion in humans by ingestion of unfermented milk containing Bifidobacterium longum’. J Dairy Sci. 79(5):750-7.
Laake K.O., et al. (2005). ‘Outcome of four weeks intervention with probiotics on symptoms and endoscopic appearance after surgical reconstruction with a J-configurated ileal-pouch-anal-anastomosis in ulcerative colitis’. Scandinavian Journal of Gastroenterology (40):43-51.
Lin MY (1991) ‘Influence of non-fermented dairy products containing bacterial starter cultures on lactose maldigestion in humans’. Journal of Dairy Science 74(1):87-95.
Hatcher G.E, Lambrecht RS. (1993) ‘Augmentation of macrophage phagocytic activity by cell-free extracts of selected lactic acid-producing bacteria’ J Dairy Sci. Sep; 76(9):2485-92.
Hilton, E., et al. (1992) ‘Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Annals of Internal Medicine, 116:353-357.
McFarland L.V. (2005) ‘Meta-analysis of probiotics for the prevention of traveller's diarrhoea’. Travel Med Infect Dis. 5(2):97-105.
Miettinen M, et al., (1996) ‘Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria’. Infect Immun. 64(12):5403-5.
Nord, C. E. et al., (1997) ‘Oral supplementation with lactic acid-producing bacteria during intake of cl****mycin’, Clinical Microbiology and Infection, 3(1):124 – 132.
Obradovic, D., et al (1996). ‘Probiotic function of the fermented milk Jogurt Plus’. FEMS Conference (Fifthe Symposium on Lactic Acid Bacteria), Holland, Sept. 8-12.
Sagen, O.B. ‘Treatment of functional disturbance in the intestine by administration of lactic acid bacteria’. 1989. Internal report.
Savard P. et al, (2011) ‘Impact of Bifidobacterium animalis subsp. lactis BB-12® and Lactobacillus acidophilus LA-5-containing yoghurt, on fecal bacterial counts of healthy adults’. International Journal of Food Microbiology, 149(1):50-7.
Sheu B.-S, et al (2002) ‘Impact of supplement with Lactobacillus- and Bifidobacterium-containing yogurt on triple therapy for Helicobacter pylori eradication’. Alimentary Pharmacology and Therapeutics. 16(9):1669–1675.
Sheu B-S (2006) ‘Pre-treatment with Lactobacillus and Bifidobacterium-containing yogurt can improve the efficacy of quadruple therapy in eradicating residual Helicobacter pylori infection after failed triple therapy’. American Journal of Clinical Nutrition. 83(4):864-869.
Shioya M, et al (2000). ‘Effect of fermented milk containing Bifidobacterium lactis FK 120 on the fecal flora and fecal properties in healthy female volunteers’. Food Health and Nutrition Research (Journal of Nutritional Food) 2000; 3:7-18.
Shioya M, et al (2000) ‘Effect of fermented milk containing Bifidobacterium lactis FK 120 on the fecal flora, with special reference to Bifidum species, and fecal properties in elderly volunteers’. Food Health and Nutrition Research (Journal of Nutritional Food), 3:33-44.
Tejada-Simon MV, et al. (1999) ‘Ingestion of yogurt containing Lactobacillus acidophilus and Bifidobacterium to potentiate immunoglobulin A responses to cholera toxin in mice’. J Dairy Sci. Apr; 82(4):649-60.
Tonucci LB (2017) ‘Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study’. Clinical Nutrition, 36(1):85–92.
Virta, P., et al. (1993) ‘The effect of a preparation containing freeze-dried lactic acid bacteria on lactose intolerance’. 1993. External report
Wang K-Y. (2004) ‘Effects of ingesting Lactobacillus and Bifidobacterium-containing yogurt in subjects with colonized Helicobacter pylori’. American Society for Clinical Nutrition 80(3):737-741.
Wildt S, et al. 2006. ‘Probiotic treatment of collagenous colitis: A randomized, double-blind, placebo-controlled trial with L. acidophilus and Bifidobacterium animalis subsp. lactis’. Inflamm Bowel Dis 12 (5): 395-401.