Bifidobacterium lactis Bi-07® is a gram-positive, anaerobic, rod-shaped bacterium which can be found in the large intestines of most mammals. This strain was recently reclassified as Bifidobacterium animalis subsp. lactis Bi-07®, but for ease it tends to be known as Bifidobacterium lactis Bi-07® (Masco et al., 2004). It may sometimes be seen named as Bifidobacterium lactis ATCC SD5220 and is a member of the Bifidobacterium lactis species.
This strain has been researched as a food supplement to examine safety when taken daily over several months, as determined by blood analysis for haematology and clinical chemistry. A paper by Cox, A. J. et al., in 2014 describes how Bifidobacterium lactis Bi-07® (in combination with Lactobacillus acidophilus NCFM®) was given to healthy active adults for 5 months. Routine haematology and clinical chemistry measurements were consistent with the placebo group, indicating this food supplement when taken over a period of months pose no safety or tolerability issues.
Survival of live cultures strains through the gut can be determined in a variety of ways, one of which is to use an in vitro colon simulator. In a study by Forssten, S. D., & Ouwehand, A. C., in 2017 an in vitro colon simulator model was used to assess the survival of strains when put through the test as single strains, or when combined as a group of strains. One of the strains tested was B. lactis Bi-07®, and its survival was equally detectable as a single strain or part of a combination with other strains. This indicates that B. lactis Bi-07® when taken as a food supplement either as a single strain or combined with other strains, should survive to reach the gut alive.
The ability of gut bacteria to modulate the immune system has been the focus of much microbiome research. Evidence suggests that probiotic bacteria may have the ability to affect certain immune responses: they may improve the response to oral vaccination; reduce the risk of certain types of infection or shorten its duration, or reduce the risk of or alleviate the symptoms of allergy and other immune-based conditions. Bifidobacterium lactis Bi-07® is one of the bacterial strains that has been used extensively in research studies to determine the role of probiotics in immune function, and the resulting evidence suggests that it may be one of the best strains for this purpose.
Bifidobacterium lactis Bi-07® was tested for its ability to stimulate a specific immunity reaction in response to vaccination. Human volunteers were orally vaccinated using cholera vaccine as a vaccination model. Over a 21 day period they were then given either a daily placebo (maltodextrin) or a daily supplement of Bifidobacterium lactis Bi-07®. Blood samples were collected on day 0, 21 and 28, to test levels of IgG (Immunoglobulin G) antibodies. It was found that those in the probiotic group displayed faster and higher IgG induction than in the control group, which suggested that there had been stimulation of a specific immune response by Bifidobacterium lactis Bi-07® (Paineau et al. 2008).
In another double-blind, placebo-controlled study, 326 eligible children (3–5 years of age) were randomly given either a placebo, a probiotic containing Lactobacillus acidophilus NCFM®, or a probiotic containing Lactobacillus acidophilus NCFM® in combination with Bifidobacterium lactis Bi-07® over a six month period.
The results indicated that, in comparison to the placebo group, both single and combination probiotics reduced incidences of fever by 53.0% and 72.7%, incidences of coughing by 41.4% and 62.1% and rhinorrhoea (runny nose) incidence by 28.2% and 58.8% respectively. Fever, coughing, and rhinorrhoea duration was decreased significantly, relative to placebo, by 32% using the single strain and 48% in the group given the strain combination, compared to the placebo group. The need for antibiotics was also reduced, relative to placebo, as was absence from school due to illness, compared with subjects receiving placebo treatment (Leyer et al., 2009).
A double-blind, randomised and controlled trial conducted by Lehtinen et al. (2014) looked at daily supplementation with Bifidobacterium lactis Bi-07® on 37 elderly subjects. The authors found beneficial effects on the immune responses of the participants, noted as an increase in phagocytic activity of both monocytes and granulocytes, one of the immunological mechanisms by which the body helps prevent itself from infections and illness.
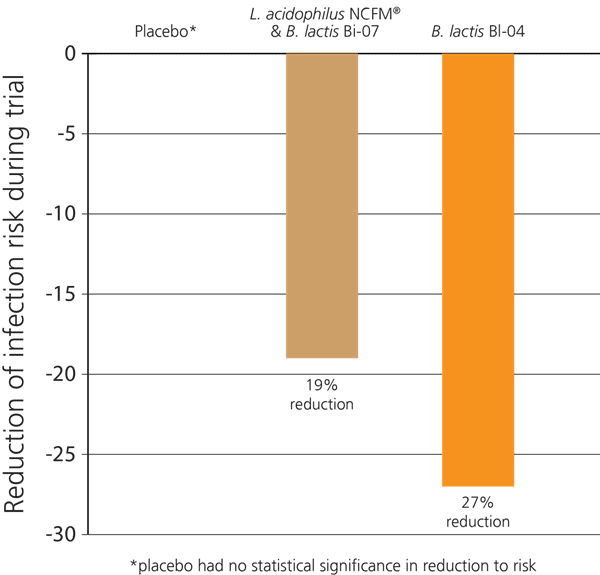
In another recent double-blind, randomised, placebo-controlled Australian study, 465 participants who exercised regularly were investigated to see if probiotics would help to support their immune function, as significant exercise can place a burden on the immune system and lead to increased incidence of coughs and colds. The subjects were split into three groups: the subjects in the first group took a supplement containing only Bifidobacterium lactis Bl-04®, those in the second group took a combination of L. acidophilus NCFM® & B. lactis Bi-07®, and those in the third group were given a placebo.
Over the 5 months of the trial it was found that the first group had a 27% decreased risk of contracting an upper respiratory infection. Those in the second group also had a decrease in their risk of infection, but it was not as significant as that seen in the first group, indicating that it was the Bifidobacterium lactis Bl-04® which offered the greatest potential benefit for immune support. However, the L. acidophilus NCFM® & B. lactis Bi-07® probiotic group also experienced improved immunity compared to the placebo group - see below (West et al., 2014).
Further relevant studies: Foligne et al (2007), Maneerat et al (2013), Wagner et al. (1997), Wagner et al. (1998), Wagner et al. (2000).
As documented throughout this database, antibiotic drugs are known to affect the composition of the gut microbiota, sometimes adversely, and research is being carried out to determine whether probiotics can help to minimise this disturbance. Bifidobacterium lactis Bi-07® has been tested for this purpose in combination with other strains of bacteria.
One such study used 51 randomised healthy subjects, who were administered with amoxicillin/clavulanate antibiotics over a period of 48 days. Subjects were divided into two groups, in which the members were given either a probiotic supplement or a placebo. Prior to antibiotic therapy, the subjects gave three faecal samples to establish their microbiota patterns when healthy, then four faecal samples were taken post antibiotic therapy for comparison. The results showed that those subjects who took the probiotic showed less disturbance in their faecal microbiota, and had a more rapid return to their pre-antibiotic microbial state (Engelbrektson et al., 2009).
A further study attempted to determine whether symptoms of diarrhoea responded more positively to higher or lower doses of probiotic bacteria. As part of the study, Bifidobacterium lactis Bi-07® was given to subjects in combination with three other strains of bacteria including B. lactis Bl-04®, L. acidophilus NCFM®, and L. paracasei Lpc-37®. The 510 participants were all suffering from diarrhoea symptoms, either antibiotic-associated diarrhoea or diarrhoea caused by Clostridium difficile infection. They were divided into three groups: subjects in the first group were given a placebo, and two treatment groups were each given the probiotic supplement of differing strengths. One group received a dose of 17 billion CFU and the other group received a dose of just over 4 billion CFU; results indicated that the higher dose of the probiotic supplement was more effective than the lower dose in alleviating diarrhoea symptoms, including the duration and incidence in a hospital setting (Ouwehand et al., 2014).
Further related study: Engelbrekston et al., (2006)
Diarrhoea is a common and unpleasant digestive symptom for all age groups and as such, as you will see from many of the other strain pages in this database, this symptom has become a key area of focus in probiotic research. It can be a particularly serious problem in children, who may become quickly dehydrated and debilitated when suffering from loose stools for prolonged periods of time. The potential for probiotics to reduce the incidence of childhood diarrhoea was evaluated in a double-blind, placebo-controlled, randomised clinical trial. A total of 243 children aged 12-36 months were recruited for the purposes of the study, and divided into two groups. One group received a placebo, and the treatment group received a probiotic supplement containing Bifidobacterium lactis Bi-07® as part of a three-strain combination (also including a Lactobacillus reuteri strain (not specified) and Lactobacillus acidophilus NCFM®). During the 14-week intervention period, a statistically significant reduction in the incidence and frequency of diarrhoea episodes was recorded in the probiotic group compared to the placebo group (Ruiz-Palacios, G. F. et al., 1999).
Bloating and distension are very common digestive symptoms which occur due to a build-up of gases in the abdominal area. This can be caused by indigestion, an overgrowth of harmful bacteria or yeasts in the intestines, or as a side effect of slow stool transit and constipation.
A placebo controlled, double blind clinical trial attempted to determine whether a probiotic supplement containing Bifidobacterium lactis Bi-07® in combination with Lactobacillus acidophilus NCFM® would alleviate bloating symptoms in the test group. A total of 57 patients with IBS (without constipation), diarrhoea and functional bloating were enrolled and randomly given a placebo or probiotic for 8 weeks. It was found that the supplementation of these probiotics significantly improved distention and bloating in the treatment group (Ringel et al., 2008).
Up to 20% of patients may experience abdominal pain post colonoscopy; this is believed to be due to alterations in the gut flora, so probiotics have been considered to be one of the best potentially helpful natural solutions.
In a recent trial, 320 randomised subjects awaiting colonoscopy were divided into two groups. Following their procedures, one group was given a probiotic containing Lactobacillus acidophilus NCFM® and Bifidobacterium lactis Bi-07®, and the other group was given a placebo capsule. Results showed a significant reduction in the duration of pain experienced by patients in the probiotic treatment group compared with placebo (D’Souza et al., 2015).
Atopic dermatitis (AD) is an increasingly common chronic inflammatory skin disease which is a cause of considerable distress, particularly in afflicted children. Solutions for prevention and treatment of this condition are the focus of a growing area of research, and as the integrity of the intestinal mucosal barrier is thought to be implicated with atopic dermatitis (AD), a role for probiotics has been considered.
In a double-blind, randomised placebo-controlled intervention using 50 children with AD, the children were given either a placebo, or a probiotic supplement containing either Lactobacillus acidophilus NCFM® or Bifidobacterium lactis Bi-07®. Clinical and immunological changes were evaluated after ingestion of the probiotic strains, and a measurable reduction in the severity of AD was observed in the Bi-07 group (Gobel et al., 2010).
Eating disorders after bariatric surgery are difficult to assess and may be under-reported.
The use of probiotics as a facilitator in the treatment of eating disorders has already been investigated as a means of modulating the microbiota-gut-brain axis.
In a randomised, double-blind placebo-controlled trial, 101 patients received either probiotic supplementation of Lactobacillus acidophilus NCFM and Bifidobacterium lactis Bi-07 or placebo for 90 days after bariatric surgery, starting on the seventh postoperative day. The Yale Food Addiction Scale (YFAS) and Binge Eating Scale (BES) were applied to assess food addiction and binge eating, respectively.
Before surgery, one-third of the patients presented with a food addiction and binge eating diagnosis. After surgery a significant effect of treatment with probiotics was observed 1 year on. Both the number of symptoms of food addiction and the binge eating score were lower in the probiotic group than in the placebo group (p=0.037 and p=0.030, respectively). (Ramos et al., 2022)
Bifidobacterium lactis Bi-07® is known to have a high lactase production activity and may potentially be able to help with lactose intolerance. This potential was demonstrated in two crossover clinical trials, in which this strain was shown to support lactose digestion in individuals with lactose intolerance. Participants were either given B. lactis Bi-07®, 4662 FCC lactase, or a placebo, whilst undertaking a lactose challenge. There was a one week washout period between each challenge. A hydrogen breath test (BHC) was used to measure the effects on lactose digestion. The probiotic group had superior BHC results in both trials. (Rasinkangas et al, 2022).
Authors: Information on this strain was gathered by Joanna Scott-Lutyens BA (hons), DipION, Nutritional Therapist; and Kerry Beeson, BSc (Nut.Med) Nutritional Therapist.
Last updated - 20th December 2022
As some properties & benefits of probiotics may be strain-specific, this database provides even more detailed information at strain level. Read more about the strains that we have included from this genus below.
Bifidobacterium lactis strains: Bifidobacterium lactis BB-12®, Bifidobacterium lactis HN019 and Bifidobacterium lactis Bl-04®.
Bifidobacterium infantis strains: Bifidobacterium infantis 35624.
Bifidobacterium breve strains: Bifidobacterium breve M-16V®.
For more information and the latest research on probiotics, please visit the Probiotic Professionals pages.
Cox et al., (2014). ‘Effects of probiotic supplementation over 5 months on routine haematology and clinical chemistry measures in healthy active adults', Eur J Clin Nutr., 68(11):1255-7. doi: 10.1038/ejcn.2014.137. Epub 2014 Jul 23.
D’Souza B. et al., (2015). ‘Randomized controlled trial of probiotics after colonoscopy’, ANZ Journal of Surgery, doi: 10.1111/ans.13225.
Engelbrekston, AL, et al (2009) 'A randomized, double blind, controlled trial of probiotics to minimize the disruption of fecal microbiota in healthy subjects undergoing antibiotic therapy'. Journal of Medical Microbiology, 58:663-670
Engelbrektson, A.L., et al., (2006). ‘Analysis of treatment effects on the microbial ecology of the human intestine. FEMS Microbiol. Ecol. 57:239-250.
Foligne, B., et al., (2007). ‘Correlation between in vitro and in vivo immunomodulatory properties of lactic acid bacteria’. World Journal of Gastroenterology 13(2):236-243.
Forssten, S. D., & A. C. Ouwehand, A. C., (2017) ‘Simulating colonic survival of probiotics in single-strain products compared to multi-strain products’. Microbial Ecology in Health and Disease, 28(1): 1378061, DOI: 10.1080/16512235.2017.1378061
Gobel et al., (2010). ‘Probiotics to young children with atopic dermatitis: A randomized placebo-controlled trial’. International Journal of Probiotics and Prebiotics, 5(2):53-59.
Lammers, K.M., (2003). ‘Immunomodulatory effects of probiotic bacteria DNA: IL-1 and IL-10 response in human peripheral blood mononuclear cells’. FEMS Immunology and Medical Microbiology 38: 165-172.
Leyer G.J. et al., (2009). ‘Probiotic effects on cold and influenza-like symptom incidences and duration in children’. Pediatrics, 124:72-179.
Maneerat S. et al., (2013). ‘Consumption of Bifidobacterium lactis Bi-07 by healthy elderly adults enhances phagocytic activity of monocytes and granulocytes’ J Nutr Sci.., 2(2):e44.
Masco L. et al., (2004). ‘Polyphasic taxonomic analysis of Bifidobacterium animalis and Bifidobacterium lactis reveals relatedness at the subspecies level: reclassification of Bifidobacterium animalis subsp. Animalis subsp. nov. and Bifidobacterium lactis as Bifidobacterium animalis subsp. Lactis subsp. Nov’. Int. J. System. Evol. Microbiol., 54(4): 1137-1143.
Ouwehand A.C. et al., (2014). ‘Probiotics reduce symptoms of antibiotic use in a hospital setting: A randomized dose response study’. Vaccine, 32(4):458-63.
Paineau D., et al., (2008). ‘Effects of seven potential probiotic strains on specific immune responses in healthy adults: a double-blind, randomized, controlled trial’. FEMS Immunology & Medical Microbiology, 53 (1):107–113.
Ramos, M.R.Z. et al., (2022). 'Probiotic Supplementation Attenuates Binge Eating and Food Addiction 1 Year After Roux-en-Y Gastric Bypass: A Randomized, Double-Blind, Placebo-Controlled Trial'. Brazilian Archives of Digestive Surgery, 35:e1659.
Rasinkangas P, et al (2022). Bifidobacterium animalis subsp. lactis Bi-07 supports lactose digestion in vitro and in randomized, placebo- and lactase-controlled clinical trials. Am J Clin Nutr. 2022 Dec 19;116(6):1580-1594. doi: 10.1093/ajcn/nqac264. PMID: 36149331.
Ringel Y. et al., (2008). ‘Probiotic bacteria Lactobacillus acidophilus NCFM® and Bifidobacterium lactis Bi-07 improve symptoms of bloating in patients with function bowel disorders (FBD)’. J. Clin. Gastroenterology,45(6):518–525.
Ruiz-Palacios G. F. et al., (1999). ‘Feeding of a probiotic for the prevention of community acquired diarrhoea in young Mexican children’. Pediatr. Res. 39(2): 104 (abstr).
Turner et al., (2017). ‘To study the effect of HOWARU protect Adult on immune response rhinoviral replication and symptoms during experimental rhinovirus infection’. ClinicalTrials.gov NCT01669603.
Wagner R. D., et al., (1997). ‘Biotherapeutic effects of probiotic bacteria on candidiasis in immunodeficient mice’. Infect. Immun., 65:4165-4172.
Wagner R.D. et al., (1998). ‘Biotherapeutic effects of Bifidobacterium spp. on orogastric and systemic candidiasis in immunodeficient mice’. Rev Iberoam Micol., 15: 265-270.
Wagner R.D. et al., (2000). ‘Effects of probiotic bacteria on humoral immunity to Candida albicans in immunodeficient bg/bg-nu/nu and bg/bg-nu/+ mice’. Rev Iberoam Micol., 17:55-59.
Wei et al., (2014). ‘Probiotic Bifidobacterium animalis subsp. lactis Bi-07 alleviates bacterial translocation and ameliorates micro inflammation in experimental uraemia’. Nephrology, 19: 500–506. doi:10.1111/nep.12272.
West N.P. et al., (2014). ‘Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy, physically active individuals’. Clinical Nutrition, 33(4):581-7.